Diagnosing the Cancer Patient
By, Chelsea Greenberg, DVM, MS, Diplomate ACVIM (oncology)
Proper diagnosis of cancer is paramount in oncology to improve patient comfort and prognosis. The growing field of oncology can appear extremely complex. However, it can be broken down into a few simple, yet extremely important questions:
What is it?
How far has it spread?
Now, what can we do about it?
What is it?
Knowing exactly what type of neoplasia is present is essential to not only select the proper treatment and formulate prognosis, but also determine probable metastatic locations. Options for tissue diagnosis often consist of diagnostic cytology or biopsy. Although both techniques are useful to diagnose cancer, they are both highly dependent on sample quality and quantity.
Diagnostic cytology – Cytology is the examination of individual cells without architectural structure. In a properly prepared slide, the cells will have a “fried egg” appearance as the nucleus and cytoplasm closely resemble the egg yolk and egg white, respectively. Acquisition of cytologic samples can be made with aspirates, scrapings, swabs, or imprints. When submitting sample to the lab, be sure to give the pathologist a brief, useful patient history so that they in return can give you the best diagnostic information possible. This also applies for diagnostic biopsy or histopathology below. When smearing slides, be sure not to press too hard and lyse the cells.
Cytology – note the “fried egg in a pan” appearance of the cells
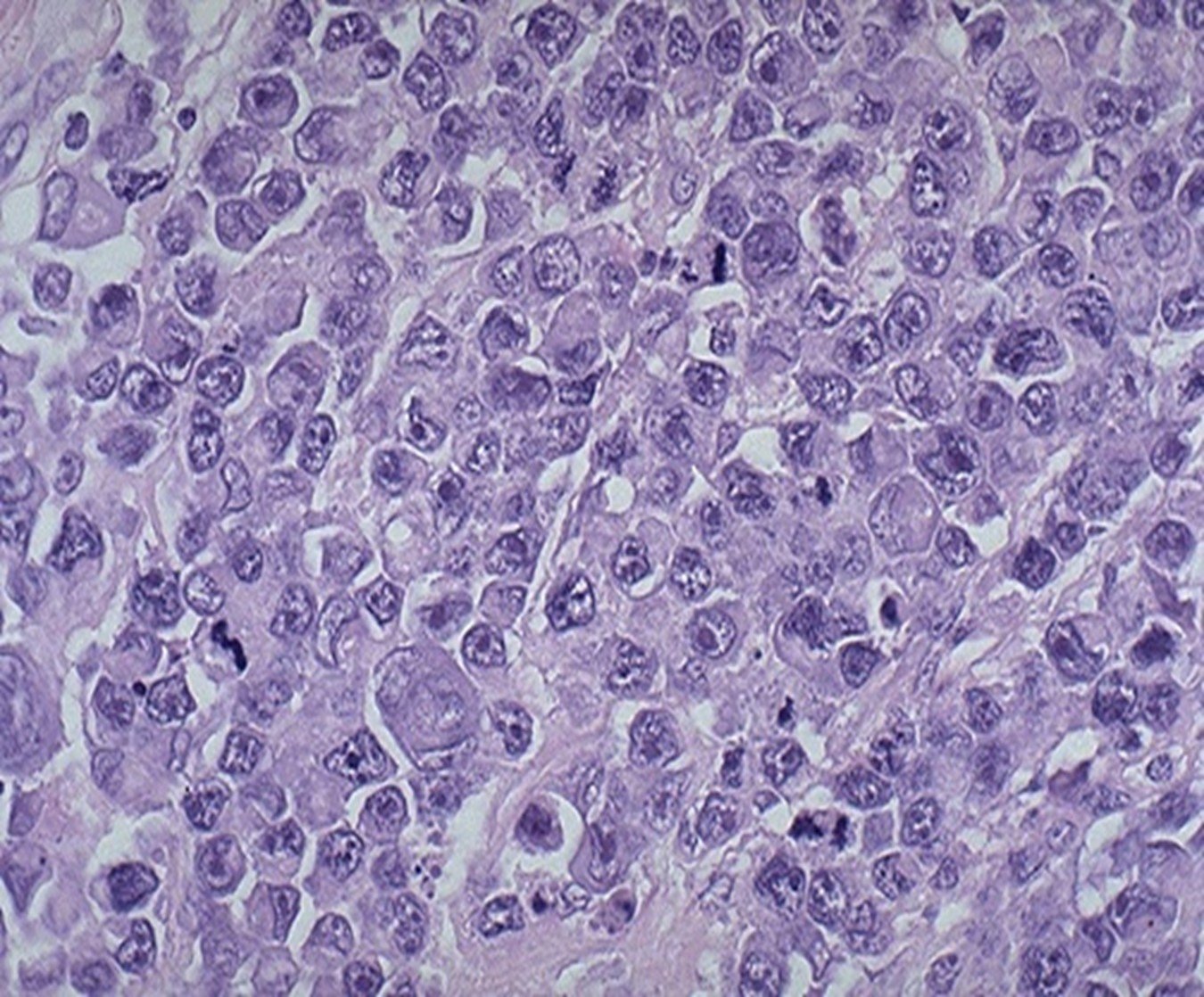
Diagnostic biopsy (histopathology) – Biopsy is the process of obtaining tissue to examine cells and the surrounding architectural structure. The cells will be surrounded by the supporting structures and will often look like “eggs in a carton” versus “egg in a pan” that are seen with cytology samples. Not only is this method the best for diagnosis, histopathology also has prognostic value, such as grading for mast cell tumors. Acquisition of biopsy samples can range from needle biopsies to complete excision.
Histopathology – note the cells are arranged within the stroma (eggs in a carton)
Types of diagnostic biopsy:
o Incisional biopsy – removal of a piece of the mass. This is an excellent diagnostic method requiring extensive margins for removal (oral tumors, vaccine associated sarcomas, etc.)
o Excisional biopsy – removal of the entire mass without intention for margins. This is not often useful in the cancer patient as initial dirty margins can be detrimental to further treatment success! Excisional biopsy can be considered in very specific instances, such as mammary tumors, distal limb mast cell tumors (where amputation can be performed to get wide margins)
Additional diagnostics – Additional diagnostics can be helpful when morphology from cytology or biopsy alone is not enough to make an accurate diagnosis. Not only can these tests assist with diagnosis, they also can assist with cancer subclassification, prognosis and treatment response.
o Flow cytometry – Flow cytometry is a technology that can sort and identify single cells that are tagged with chosen reagents (such as CD3). The cells are suspended in solution and light scatter and fluorescence are detected by photodiodes or photomultiplier tubes. Detectors then measure the wavelength of the light in the forward direction (forward scatter) which can indicate cell size and from 90º (side scatter) which indicates granularity or complexity of the cell. The results are then plotted on a scatterplot for evaluation. The forward scatter is plotted on the x-axis and the side scatter is plotted on the y-axis (see below). This technique can be helpful at sub classifying lymphomas1 or differentiating thymoma versus lymphoma2 in mediastinal masses.
Lucroy MD, et al. Evaluation of an autologous cancer vaccine for the treatment of metastatic canine hemangiosarcoma:
a preliminary study. BMC Vet Res. 2020 Nov 18;16(1):447. doi: 10.1186/s12917-020-02675-y
o Immunostaining - Immunostaining is a technique for assessing protein expression on the cell surface and can aid in diagnosis where morphology is questionable. Immunostaining is often referred to as “special stains”. This technique can be used on fresh or fixed tissues. Primary antibodies are used to bind to the desired protein. A secondary antibody is then attached to the primary antibody. Often a labeling protein is attached to the secondary antibody. The labeling protein helps identify the expression of the desired protein. Immunocytochemistry is the staining of cytology samples and immunohistochemistry is the staining of histopathology samples. The biggest limitation of immunostaining is cellular mutations. If the cells can mutate a particular protein expression, the desired antibodies may not bind well which can give false negative staining results. It is important to communicate with clients that although immunophenotyping can often help arrive at a diagnosis, due to the cellular mutations, it may not in all cases.
o Polymerase chain reaction (PCR) - This technique amplifies a DNA or RNA fragment of interest so that it can be detected. There are multiple variations of this technique that are used for different diagnostic tests.
Step 1: Denaturation (high temperature)
Melting of the double stranded DNA into single strands.
Step 2: Annealing (lower temperature)
Primers form ionic bonds with single stranded DNA target
Step 3: Extension
Extension of the new DNA strands from the primers
Cycle repeats
The PCR products can then be used for other genetic analyses.
PARR is a common use of the PCR technology to look for clonal expansion of B or T lymphocytes. PARR uses PCR to detect antigen receptor rearrangements and can be used to help diagnose lymphoma in dogs and cats. PARR can help facilitate or exclude the diagnosis of lymphoma and can sometimes subclassify lymphoma into B or T cell.3-5
Next Generation Sequencing (NGS) is a high volume parallel genomic sequencing technology that utilizes bridging PCR technology to investigate whole genomes or multiple targeted regions at one time. This technique offers a fast method of genetic profiling and can be applied to blood (liquid biopsy) as well as tumor tissue.
Liquid biopsy – Liquid biopsies are performed on blood samples for detection and isolation of circulating cancer cells, cancer DNA, or cancer proteins. This technology is currently being evaluated as a screening tool for early cancer detection and is also commercially available.
Tumor tissue – This use of NGS is targeted at therapeutics versus diagnostics. This technology evaluates genetic mutations that may be actionable targets for cancer therapy.
How far has it spread?
Diagnostic imaging:
Diagnostic imaging plays an essential role in the diagnosis and management of the cancer patient. Once the cancer diagnosis is made, proper imaging choices can be made for cancer staging. With many recent technological advances, many imaging modalities have been developed for use in the veterinary patient.
Radiography – Radiography has been the basis of cancer imaging for many years. Availability, low cost, and speed are three advantages of conventional radiography. Radiography also offers more of the “big picture” versus more advanced imaging as the resolution is not very high. Often conventional radiography is followed by advanced imaging for more detailed staging and treatment planning. Three view (right lateral, left lateral, and ventrodorsal views) thoracic radiographs are commonly used to stage a variety of cancers.
Ultrasonography – Ultrasonography offers the ability to evaluate the architecture of organs in higher detail so can be a useful modality to evaluate the abdomen. Ultrasound is also useful for imaging organs when cavitary effusion is present, where loss of detail on radiographs can make evaluation difficult. Ultrasonography can also be used to help guide needles for diagnostic biopsy or cytology.
Computed tomography - Computed tomography (CT) offers more detail than conventional radiography and ultrasound and is more sensitive at identifying smaller structures. Because the image is computer generated and is oriented in transverse “slices”, there is no superimposition of structures, and the image contrast resolution is much higher than conventional radiography. Some examples where CT is useful is identifying pulmonary nodules, lymphadenopathy in the thorax and abdomen, and evaluation of the skull. Contrast-enhanced CT images can enhance tumor detection as well as extent of the tumor.
Magnetic Resonance Imaging – Magnetic Resonance Imaging (MRI) produces images that look similar to CT; however, MRI does not use radiation like CT. MRI measures the random motion of water molecules in tissues that have been manipulated by a magnet. The images from MRI are superior for central nervous system imaging as well as other soft tissue structures. Because MRI is not fast enough to image fast moving tissues, its use for evaluating the lungs and heart is limited.
Nuclear medicine (scintigraphy) – Nuclear scintigraphy uses radiopharmaceuticals to localize to the area of interest through normal metabolic processes in the patient. Tissue uptake of the radiopharmaceutical is seen in areas of high metabolism which can indicate cancerous lesions. Images produced with nuclear scintigraphy do not provide great detail (low resolution). Due to the low resolution, scintigraphy is limited to generalized lesion detection/screening versus specific lesion location information. Also, tissue uptake of the chosen radiopharmaceutical can help predict treatment efficacy for certain tumor types (such as Technetium-99 for Samarium efficacy for primary bone tumors in dogs). If any suspicious lesions are found, additional imaging modalities are needed to further characterize those areas (such as CT or MRI). In human medicine PET CT is widely available for cancer patients which combines the images of nuclear medicine with those of a CT scan. A few facilities have PET CT available for use in pets.
(B) Nuclear scintigraphy (PET) transverse image of a right auricular appendage mass in a dog with hemangiosarcoma (C) Fused PET-CT transverse image.6
Although diagnostic imaging can be helpful with tumor detection, biopsy guidance, and treatment planning, it is not specific for etiology. The only way to diagnose cancer is with histopathology or a combination of histopathology and cytology.
Now, what can we do about it?
Good question, however, it will have to wait for another day! Even before treatment takes place, an accurate diagnosis and appropriate staging will help set clear client expectations and your patients up for success.
References:
1Riondato, Fulvio, and Stefano Comazzi. Flow Cytometry in the Diagnosis of Canine B-Cell Lymphoma. Frontiers in veterinary science vol. 8 600986. 19 Mar. 2021, doi:10.3389/fvets.2021.600986
2Lana S, Plaza S, Hampe K, Burnett R, Avery AC. Diagnosis of mediastinal masses in dogs by flow cytometry. J Vet Intern Med. 2006 Sep-Oct;20(5):1161-5. doi: 10.1892/0891-6640(2006)20[1161:dommid]2.0.co;2. PMID: 17063710.
3Ehrhart, EJ, Wong, S, Richter, K, et al. Polymerase chain reaction for antigen receptor rearrangement: Benchmarking performance of a lymphoid clonality assay in diverse canine sample types. J Vet Intern Med. 2019; 33: 1392– 1402. https://doi.org/10.1111/jvim.15485
4Carrasco V, Rodríguez-Bertos A, Rodríguez-Franco F, et al. Distinguishing Intestinal Lymphoma From Inflammatory Bowel Disease in Canine Duodenal Endoscopic Biopsy Samples. Veterinary Pathology. 2015;52(4):668-675. doi:10.1177/0300985814559398
5Peter F. Moore, Jennifer C. Woo, William Vernau, Sandra Kosten, Petra S. Graham. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Veterinary Immunology and Immunopathology, Volume 106, Issues 3–4, 2005, Pages 167-178, ISSN 0165-2427, https://doi.org/10.1016/j.vetimm.2005.02.014.
6Borgatti, A, et al. Evaluation of 18-F-fluoro-2-deoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) as a staging and monitoring tool for dogs with stage-2 splenic hemangiosarcoma – A pilot study. PLOS ONE. 12. e0172651. 10.1371/journal.pone.0172651.